| CPC C01D 15/02 (2013.01) [B01D 61/58 (2013.01); B01J 39/16 (2013.01)] |
| AS A RESULT OF REEXAMINATION, IT HAS BEEN DETERMINED THAT: |
| The patentability of claims 8 and 18 is confirmed. |
| Claims 1-7 and 9-17 are cancelled. |
| New claims 19-53 are added and determined to be patentable. |
|
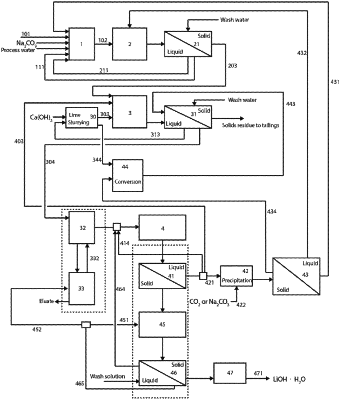
[ 19. A method for recovering lithium hydroxide from a raw material containing lithium, selected from spodumene, petalite or lepidolite or mixtures thereof, wherein the method comprises:
pulping the raw material containing lithium in the presence of water and an alkali metal carbonate for producing a first slurry containing lithium;
leaching the first slurry containing lithium in a first leaching step at an elevated temperature for producing a second slurry containing lithium carbonate;
leaching at least a fraction of the second slurry in a second leaching step in a first aqueous solution containing an alkali earth metal hydroxide for producing a third slurry containing lithium hydroxide,
separating, in a solid-liquid separation step using a solid-liquid separation unit, the third slurry into solids and a second aqueous solution containing lithium hydroxide
purifying, in a purifying step that removes dissolved impurities using a purification unit, the second aqueous solution, thus providing a purified solution containing lithium hydroxide and at least some amount of dissolved impurities,
crystallizing, in a crystallizing step using a crystallization unit, the purified solution containing lithium hydroxide to recover lithium hydroxide monohydrate, wherein a third aqueous solution containing at least some amount of dissolved impurities is obtained during the crystallizing step; and
recycling the third aqueous solution to one of the previous steps, with at least a fraction of the third aqueous solution being recycled to the second leaching step, wherein the recycling of the third aqueous solution to the second leaching step results in lithium hydroxide with fewer impurities.]
|
|
[ 20. The method according to claim 19, wherein the purifying step is performed by ion exchange.]
|
|
[ 21. The method according to claim 19, wherein the purifying step is performed using a cation exchange resin.]
|
|
[ 22. The method according to claim 21, wherein the purifying step is performed using a selective cation exchange resin.]
|
|
[ 23. The method according to claim 19, wherein the purifying step is performed using an iminodiacetic acid chelating cation exchange resin.]
|
|
[ 24. The method according to claim 19, wherein the purifying step is performed using an aminophosphonic acid chelating cation exchange resin.]
|
|
[ 25. The method according to claim 19, wherein the purifying step is performed by a membrane separation.]
|
|
[ 26. The method according to claim 19, wherein the purifying step is performed by a membrane separation and then an ion exchange.]
|
|
[ 27. The method according to claim 19, wherein the first leaching step is carried out at a temperature of 160 to 250° C. and at a pressure of 10 to 30 bar.]
|
|
[ 28. The method according to claim 26, wherein the first leaching step is carried out at a temperature of 200 to 220° C. and at a pressure of 15 to 25 bar.]
|
|
[ 29. The method according to claim 19, wherein the solid-liquid separation unit is distinct from the purification step.]
|
|
[ 30. The method according to claim 19, wherein the purification unit is distinct from the crystallization step.]
|
|
[ 31. The method according to claim 19, wherein the purification unit is a membrane separation unit.]
|
|
[ 32. The method according to claim 19, wherein the purification unit is an ion exchange unit.]
|
|
[ 33. The method according to claim 19, wherein the purification unit is a membrane separation unit and an ion exchange unit.]
|
|
[ 34. The method according to claim 19, wherein the dissolved impurities are dissolved ion impurities.]
|
|
[ 35. The method according to claim 19, wherein the first slurry does not contain an acid.]
|
|
[ 36. The method according to claim 19, wherein the first slurry is substantially sulphate free.]
|
|
[ 37. The method according to claim 19, wherein the third slurry does not contain an acid.]
|
|
[ 38. The method according to claim 19, wherein the third slurry is substantially sulphate free.]
|
|
[ 39. The method according to claim 19, wherein the alkali metal carbonate used in the pulping step is selected from sodium and potassium carbonate.]
|
|
[ 40. The method according to claim 19, wherein the second slurry is separated into obtained second solids and obtained second solution and at least a fraction of the obtained second solution is recycled to the pulping step.]
|
|
[ 41. The method according to claim 19, wherein the alkali earth metal hydroxide used in the second leaching step is selected from calcium and barium hydroxide.]
|
|
[ 42. The method according to claim 19, wherein the third aqueous solution, before being recycled to the second leaching step, is mixed with an alkali earth metal hydroxide in a separate slurrying step.]
|
|
[ 43. The method according to claim 19, wherein the second leaching step is carried out at a temperature of 10-100° C. and at atmospheric pressure.]
|
|
[ 44. The method according to claim 19, wherein the purifying step includes use of a membrane and then use of an ion exchange.]
|
|
[ 45. The method according to claim 19, wherein at least a fraction of the third aqueous solution is recycled to the pulping step.]
|
|
[ 46. The method according to claim 19, wherein at least a fraction of the third aqueous solution is used in a lithium precipitation step, wherein the third aqueous solution is reacted with at least one of a carbon dioxide or an alkali metal carbonate in order to form a lithium carbonate slurry.]
|
|
[ 47. The method according to claim 19, wherein the solids obtained in the crystallization step are subject to a washing solution in a washing step, and where used washing solution is separated from the recovered lithium hydroxide monohydrate and at least a fraction of the used washing solution is recycled to at least one of the washing step, the purifying step, the crystallization step, or a regeneration step preceding the purifying step.]
|
|
[ 48. The method according to claim 19, wherein the alkali metal carbonate used in the pulping step is at least partly composed of sodium carbonate.]
|
|
[ 49. The method according to claim 19, wherein the second slurry is separated into obtained second solids and obtained second solution and at least a fraction of the obtained second solution is recycled to the pulping step.]
|
|
[ 50. The method according to claim 19, wherein the alkali earth metal hydroxide used in the second leaching step is calcium hydroxide.]
|
|
[ 51. The method according to claim 19, wherein the second leaching step is carried out at a temperature of 20-60° C., and at atmospheric pressure.]
|
|
[ 52. The method according to claim 19, wherein the second leaching step is carried out at a temperature of 20-40° C., and at atmospheric pressure.]
|
|
[ 53. The method according to claim 19. wherein the purifying step includes purifying by use of at least one of an ion exchange or a membrane.]
|